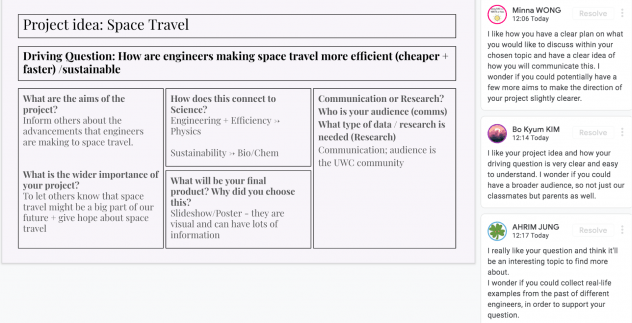
This week we finished up our CREST project and our reflection presentation. My CREST project was about “How engineers are making space technology more efficient?”, and I really enjoyed spending my time on it.
Overall I think that my project was successful as I managed to meet my main aim, which was to inform others about the advancements that engineers are making to space travel. I definitely think that my project will be able to educate others about how space travel has been advancing, and how it might be in the future. Another reason why I believe my project is successful is because the information is presented in an engaging manner – a Prezi presentation. In my presentation, the information is presented in a concise manner, and it contains many visuals, hence it will be able to engage my audience well.
However, there are quite a few things that didn’t go as planned, hence diminishing the success of my project. Firstly, I found it hard to find relevant research on my topic, more specifically, on how engineers are making the fuel used in space technology more efficient. This led me to spend a lot of time on research, hence I ended up having less time to put together my final project, and it was very rushed, therefore my project was not at the best level it could be at. I also think I could have improved my communication, because I found it quite hard to cut down on words and make my presentation concise, therefore I ended up removing a lot of information from my presentation. This means that some scientific information in my presentation is not explained properly, the reasoning behind my inferences are not thoroughly explained. This might make it harder for my audience to understand my presentation, hence diminishing the success of my project.
This project has led me to learn a lot of things about the wider world, and also about myself. From this project, I have a deeper understanding of how rockets provide propulsion for a spacecraft, and also about the evolution of space technology and how engineers can improve it to become cheaper and more efficient. For example, by using reusable spacecrafts, engineers will be able to significantly reduce costs and waste, hence increasing efficiency. This is because normal spacecrafts are expensive to build, and are thrown away after each flight, hence by using reusable spacecrafts, a new spacecraft don’t have to build for every launch, therefore reducing costs and waste.
From this project, I learn a lot about myself as well, for example, I learnt that time management is a skill I need to work on, I actually left this project for the last minute, hence it was very rushed, and it wasn’t the best level it could have been. If I had made a plan and managed my time properly, then my project would have been much better. On the other hand, I also learnt that I am willing to learn, and enjoy learning more about things that I am interested in. Since I was interested in this topic – space technology – I found it easy to be motivated to do work, despite not having much time to finish my project.
The information that I have provided to the audience that view my presentation will allow them to have a deeper understanding of how space technology works, some challenges that engineers face in relation to space technology, and also about how space technology has improved through the years. In my presentation, I also talked about how engineers might make space technology more efficient in the future, hence this allows the audience to anticipate a future where space technology is more efficient, and they will also know potential ways in which space technology might develop.
If I were to do this project again, then I definitely would put much more effort into and actually make a plan of how to managed my time efficiently, so that I don’t end up doing the project at the last minute. I also think that I would a different method of presenting my work, such as a video documentary. This is so that I will be able to convey more information to my audience in an engaging manner. I would also do more research so that the explanations provided in my presentation would be more thorough and easy to understand.
If I were to develop this project in the future, then I would do research on how space technology has improved from now, and limitations that prevent engineers form making space travel more efficient and cheaper. I would also look into the different materials that can be used to create space technology and how that will affect the efficiency of a spacecraft.
Overall, I definitely enjoyed spending time on this project since this topic – space technology – is one that interests me a lot. This is because aerospace engineering is a very interesting career that I might pursue in the future. I hope that there will be cheaper and more efficient space technology being used in the future, and if not, I hope that I will be able to play a part in making space technology more efficient.